The gut microbiome is intricately involved in many of our bodily functions.
As we continue to better understand the many roles that the gut microbiome plays, research is showing the microbiome’s contributions to both health and disease are even more significant than previously thought. But how is it involved exactly? And why is this important? Let’s take a look at a few of your gut microbe’s key functions which have a major influence on your health and wellbeing.
Understanding how your gut microbiome can influence your health
You can see how your microbiome may be influencing your health by looking at what substances the bacteria in your gut have the potential to produce. These substances, known as microbial metabolites, can influence your bodily functions. Some metabolites have been associated with health benefits while others have been associated with disease.
Several microbially produced metabolites have been implicated in the development of common health conditions, such as:
- inflammatory bowel disease (IBD)1
- obesity2
- heart disease2
- type 2 diabetes3
- and many more!
Interested to know what your gut microbes are doing? Find out more.
Microbial metabolites illuminated
Interestingly, you have some control over the production of these microbial metabolites through the foods that you eat and can therefore influence how your gut microbiome interacts with your overall health.
If you are eating a diet that is high in a diverse range of fibres, there is a greater chance that your microbiome will have sufficient levels of fibre-degrading bacteria4. When your gut microbes use fibre as a fuel source, they typically produce beneficial metabolites which can have a positive influence on your health5.
Short chain fatty acids (SCFAs) such as acetate, propionate and butyrate are produced when gut bacteria break down fibre from the foods you eat6. Research shows these metabolites are incredibly important for our health because they help5,7,8.
- control blood glucose levels
- regulate appetite
- feed gut cells
- balance the immune system
- reduce inflammation
At healthy levels, these metabolites can help keep your gut functioning appropriately so that you are less likely to experience certain health complications. On the flip side, low levels of SCFA production, specifically butyrate, can lead to gut inflammation which has been implicated in individuals with Inflammatory Bowel Disease (IBD)9,10.
Although most beneficial metabolites are produced from the fibre we eat some beneficial metabolites are produced from protein. For example, 3-indolepropionic acid (IPA) is a strong antioxidant produced by some gut bacteria when they break down tryptophan, which is found in milk, cheese, eggs, fish and meat. Research has shown that average to high levels of IPA can help protect your nervous system from damage11 and play a role in preventing type 2 diabetes12 while low levels of IPA have been shown to contribute to the development of type 2 diabetes13. However, even these beneficial protein-consuming bacteria seem to do better if you are following a diet that is high in fibre with research showing that consuming foods high in dietary fibre, and in particular rye, was linked to increased IPA producing bacteria in the gut13.
If you are eating a diet that is low in fibre this means you will have a greater chance of having excessive levels of protein or mucin degrading bacteria. Mucins make up the mucus layer protecting your gut barrier and excessive mucin degrading bacteria can lead to this protective barrier being compromised leaving your gut exposed to damage.
If your gut microbiome is geared to using protein rather than fibre, this can prevent the production of more beneficial metabolites and can also lead to excessive production of certain detrimental metabolites.
An example of a metabolite associated with poor health outcomes is the excessive production of hydrogen sulphide:
Hydrogen sulphide is a gas your gut bacteria produce when they break down sulphur-containing amino acids found in foods such as eggs, meat, and fish. At low to average levels, hydrogen sulphide is an essential metabolite that can play a beneficial role by acting as an energy source for gut cells14. However, at high levels, hydrogen sulphide can disrupt the gut barrier and promote inflammation with elevated levels of hydrogen sulphide in the gut thought to play a role in Inflammatory Bowel Disease15.
Eating a low fibre diet can encourage your gut microbes to utilise sulphur-containing amino acids as an energy source, which can lead to the excessive production of hydrogen sulphide. This means that even if your microbiome has lots of bacteria with the ability to produce hydrogen sulphide you can prevent them from actually producing excessive amounts of hydrogen sulphide by feeding them plenty of fibre.
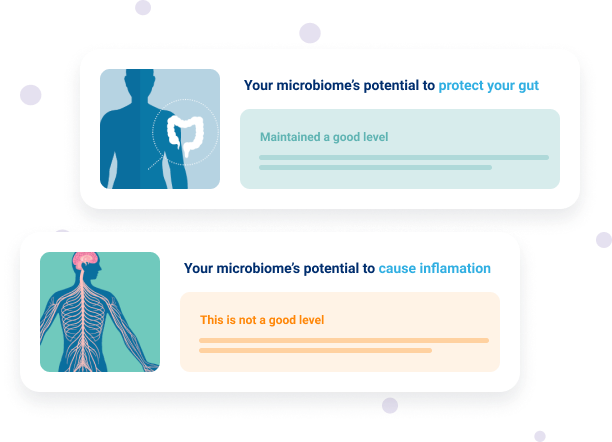
Explore your microbiome’s potential to produce certain metabolites with Insight™
Measuring your gut microbiome
So, if our health is so dependent on varying levels of these microbial substances, how do we know how much our gut microbes are potentially producing?
The use of gut microbiome analysis to measure the microbial community in your gut allows for the accurate assessment of bacterial species that produce metabolites, such as SCFAs, IPA and hydrogen sulphide. This allows you to see if your gut microbiome has sufficient levels of bacteria that have the potential to produce beneficial metabolites – and to identify what changes may be required to optimise the health of your gut.
For example, if your gut microbiome report shows a lower level of butyrate-producing bacteria and an elevated level of hydrogen sulphide producing bacteria you will receive recommendations to encourage the production of beneficial butyrate and limit the production of excessive levels of hydrogen sulphide. Increasing your resistant starch intake can achieve both of these goals. This is because consuming foods high in resistant starch has been shown to increase levels of butyrate production4,16 while laboratory studies show resistant starch to also reduce excessive levels of hydrogen sulphide production15.
Sources high in resistant starch which are provided in your report include:
- lentils
- peas
- beans
- cooked and cooled potatoes
- rolled oats
Explore your unique gut microbiome and how you may respond to various diet interventions with Insight™.
As we continue to uncover more about the gut microbiome and its associations with human health, scientists and healthcare practitioners are finding that a more targeted and personalised approach to managing overall health is beneficial when supporting your unique gut microbiome.
To begin your journey to better gut health using gut microbiome analysis, you can order an Insight™ test and work with a practitioner to assist with personalised dietary interventions. Don’t have a practitioner? A Microbiome Coach can help guide you in the right direction!
This microbiome test is not intended to be used to diagnose or treat medical conditions. A full disclaimer is available here
References
Kushkevych I, Kotrsová V, Dordević D, et al. .
Hydrogen Sulfide Effects on the Survival of Lactobacilli with Emphasis on the Development of Inflammatory Bowel Diseases. .
Biomolecules, 2019; 9(12):752. DOI: . Doi: https://doi.org/10.3390/biom9120752
Chen S, Henderson A, Petriello M, et al. .
Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction..
Cell Metabolism, 2019;1141-1151.E5.. Doi: https://doi.org/10.1016/j.cmet.2019.08.021
Cani P. D., Amar J, Iglesias M. A., et al. .
Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. .
American Diabetes Association, Diabetes, 2007; 56(7): 1761-1772. DOI: . Doi: https://doi.org/10.2337/db06-1491
Brouns F, Kettlitz B, Arrigoni E. .
Resistant starch and “the butyrate revolution”. .
Science Direct, 2002;251-261. DOI: . Doi: https://doi.org/10.1016/S0924-2244(02)00131-0
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. .
From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. .
Cell, 2016;165, 1332–1345. DOI: . Doi: https://doi.org/10.1016/j.cell.2016.05.041
den Besten, G. et al. .
The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. .
Lipid Res. 2013;54, 2325–2340. DOI: . Doi: https://doi.org/10.1194/jlr.R036012
Morrison, D. J. & Preston, T. .
Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. .
Gut Microbes, 2016;7, 189–200. DOI: . Doi: https://doi.org/10.1080/19490976.2015.1134082
Rios-Covian D, Ruas-Madiedo P, Margolles A, et al. .
Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. .
Front. Microbiol., 2016. DOI: . Doi: https://doi.org/10.3389/fmicb.2016.00185
Cushing K. Alvarado D. Ciorba M. .
Butyrate and Mucosal Inflammation: New Scientific Evidence Supports Clinical Observation. .
Clinical and Translational Gastroenterology, 2015. DOI: . Doi: https://doi.org/10.1038/ctg.2015.34
Emilio J Laserna-Mendieta, Adam G Clooney, Julián F Carretero-Gomez, et al. .
Determinants of Reduced Genetic Capacity for Butyrate Synthesis by the Gut Microbiome in Crohn’s Disease and Ulcerative Colitis. .
JCC, 2018; Pages 204–216. DOI: . Doi: https://doi.org/10.1093/ecco-jcc/jjx137
Bendheim, P. E. et al. .
Development of indole-3-propionic acid (OXIGONTM) for alzheimer’s disease. J. .
Mol. Neurosci, 2002;19, 213–217. DOI: . Doi: https://doi.org/10.1007/s12031-002-0036-0
12. de Mello, V. D. et al. .
Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. .
Sci. Rep, 2017; 7, 46337. DOI:. Doi: https://doi.org/10.1038/srep46337
Tuomainen, M. et al. .
Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. .
Nutr. Diabetes, 2018; 8, 35. DOI: . Doi: https://doi.org/10.1038/s41387-018-0046-9
Wallace J. Motta J. Buret A. .
Hydrogen sulfide: an agent of stability at the microbiome-mucosa interface. .
Gastrointestinal and liver physiology, 2018. DOI: . Doi: https://doi.org/10.1152/ajpgi.00249.2017
Chu K. Yao. .
Modulation of colonic hydrogen sulfide production by diet and mesalazine utilising a novel gas-profiling technology. .
Gut Microbes, 2018; 9(6). DOI: . Doi: https://doi.org/10.1080/19490976.2018.1451280
McOrist A, Miler R., Bird A. R et al. .
Fecal Butyrate Levels Vary Widely among Individuals but Are Usually Increased by a Diet High in Resistant Starch..
The Journal of Nutrition, 2011; 883–889. DOI: . Doi: https://doi.org/10.3945/jn.110.128504


