Trimethylamine: A link between the gut microbiome and metabolic disease
Author: Anita Tait
February 2021

In the world of microbiology and nutrition science, there has been an explosion of new research linking the gut microbiome to health and disease. This growing interest in the gut microbiome stems from compelling new discoveries made possible due to advancements in technology. These advancements allow us to measure the billions of microbes living within our gut as well as their function which most importantly allows us to understand how these microbes influence our health.
The gut microbiome and TMA
One of these discoveries is the microbial metabolite, Trimethylamine (TMA). TMA is a microbial metabolite that is produced by our gut bacteria and has been shown to be associated with chronic health conditions such as cardiovascular disease and diabetes.
What is the clinical relevance of TMA?
Once TMA is produced by the gut microbiome, it is transported from the gut to the liver where it is converted to trimethylamine oxide (TMAO). TMAO has been shown to be involved in blood sugar control, atherosclerosis, platelet aggregation and inflammation1 (see Fig.1).
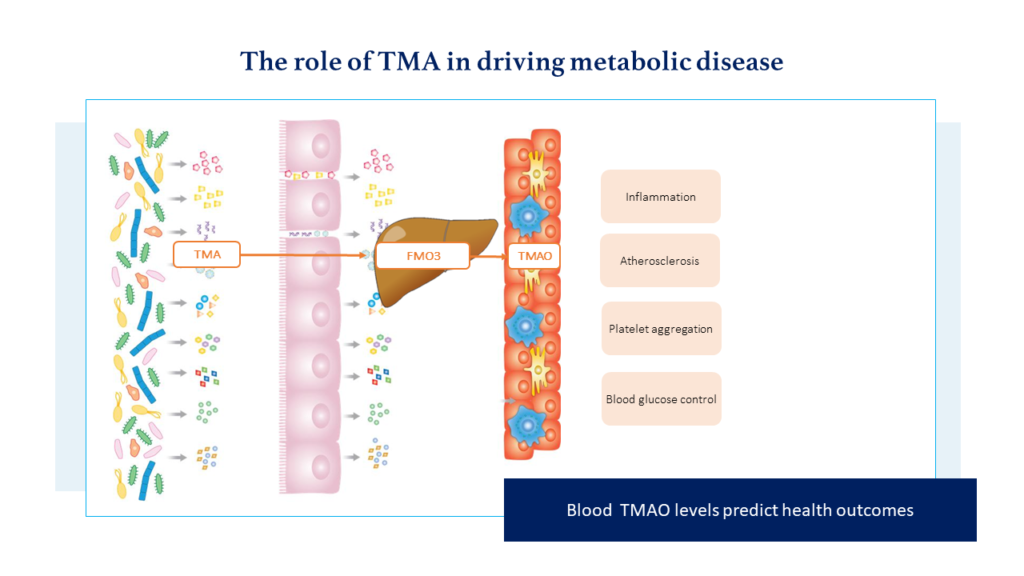
In addition, we now have a strong body of evidence that suggests blood TMAO levels can predict health outcomes across a range of metabolic health conditions. When comparing those with the highest to the lowest blood TMAO levels, a series of meta-analyses reported a 47% increase in all-cause mortality2, a 62% increase in major cardiovascular events3, an 89% increase in Diabetes Mellitus risk4 and a 0.27mg/L increase in the inflammatory marker CRP5.
How does diet have a role in TMA production?
We know that what we eat plays an important role in the production of TMA within the gut microbiome. While we have understood for some time that microbes are able to produce TMA from both choline and carnitine, recent research has shown that dietary carnitine is the principal driver of plasma TMAO levels6-7. The amino acid, carnitine is found in many dietary sources, with the richest source being red meats, such as kangaroo and beef8.
In a study by Wang and colleagues,6 a red meat diet intervention (2x serves per day) resulted in a 3-fold increase in mean fasting TMAO plasma levels when compared to white meat or non-meat diet. This increase in TMAO levels translates to a very clinically significant 4.6% increase in relative risk of all-cause mortality. Of note, the response was highly individual with some participants showing up to a 10-fold increase in TMAO blood levels in response to the red meat diet. The role of the microbiome in determining this individualised response was confirmed by a 2020 study7 which highlighted the role of the microbiome in determining the impact of dietary carnitine on plasma TMAO levels.

Find out more with our TMA Clinical Reference Guide. Download now.
What dietary interventions can reduce TMAO production?
For those that have a high potential to produce TMA in their gut microbiome, reviewing dietary carnitine intake may be warranted However, reducing red meat is not a one size fits all approach and should be considered in the context of the individual. Red meat can be an important dietary source of key vitamins and minerals, such as vitamin B12, iron and zinc, whose intake can be especially critical in young children, pregnant women and the elderly.
The Heart Foundation provides useful guidelines of aiming for less than 350g of red meat per week (cooked weight). Replacing red meat with pulses, legumes, nuts and seeds can also reduce carnitine intake while still providing good sources of iron and zinc.
Another dietary intervention to consider is increasing the intake of vegetables from the Brassica family, such as broccoli, kale, cabbage, cauliflower and Brussel’s sprouts. These vegetables contain the bioactive metabolites indole-3-carbinol (I3C) and its dimer diindolylmethane (DIM) which have been noted to inhibit the FMO3 enzyme which converts TMA to TMAO in the liver9-10. Increasing these types of vegetables will also provide the body with many important vitamins and minerals as well as a good source of prebiotic fibre.
Applying Clinical Interventions
Although it has long been understood that excessive red meat intake is associated with cardiovascular disease, recent scientific advancements have made the role of the microbiome clear in this process. Therefore, assessing a patient’s capacity to produce TMA in their microbiome allows for further targeted dietary interventions to more effectively manage an individual’s personal cardiovascular risk factors.
Find more exclusive practitioner resources on the clinical resources page.
About the author

Anita Tait
Anita is an Accredited Practicing Dietitian, Pharmacologist and Clinical Application Specialist at Microba with experience in the medical and private practice space. Anita has always been fascinated with human physiology, food and biochemistry, and is now delving into the exciting world of the gut microbiome.
References:
1.Duttaroy, A. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients, 2021; 144. https://doi.org/10.3390/nu13010144
2.Farhangi, M. Gut microbiota-dependent trimethylamine N-oxide and all-cause mortality: Findings from an updated systematic review and meta-analysis. Nutrition, 2020; 110856. https://doi.org/10.1016/j.nut.2020.110856.
3.Heianza, Y., Ma, W., Manson, J., Rexrode, K, & Qi, L]. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta‐analysis of prospective studies. Journal of the American Heart Association, 2017. https://doi.org/10.1161/jaha.116.004947
4.Zhuang R, Ge X, Han L, Yu P, Gong X, Meng Q, Zhang Y, Fan H, Zheng L, Liu Z, Zhou X. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obesity Reviews, 2019 Jun;20(6):883-894. DOI: 10.1111/obr.12843.
5.Farhangi, M. & Vajdi, M. Novel findings of the association between gut microbiota–derived metabolite trimethylamine N-oxide and inflammation: results from a systematic review and dose-response meta-analysis. Critical Reviews in Food Science and Nutrition, 2020;2801-2823. DOI: 1080/10408398.2020.1770199
6.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM, Hazen SL. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. European Heart Journal, 2019;583-594. DOI: 10.1093/eurheartj/ehy799
7.Wu, WK., Panyod, S., Liu, PY. et al. Characterization of TMAO productivity from carnitine challenge facilitates personalized nutrition and microbiome signatures discovery. Microbiome, 2020;162. DOI: 1186/s40168-020-00912-y
8.Knuttel-Gustavsen, S. & Harmeyer, J. The determination of L-carnitine in several food samples. Food Chemistry, 2007;793-804. DOI: 10.1016/j.foodchem.2007.01.058
9.Cashman JR, Xiong Y, Lin J, Verhagen H, van Poppel G, van Bladeren PJ, Larsen-Su S, Williams DE. In Vitro and In Vivo Inhibition of Human Flavin-Containing Monooxygenase Form 3 (FMO3) in the Presence of Dietary Indoles. Biochemical Pharmacology, 1999; 1047-1055. DOI: 10.1016/s0006-2952(99)00166-5.
10.Chen, S., Henderson, A., Petriello, M. et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metabolism, 2019; 1141-1151. DOI: 1016/j.cmet.2019.08.021