Medications and the gut microbiome: Much more than just antibiotics
Author: Dr Paula Smith-Brown and Dr Kaylyn Tousignant
May 2021

Have you considered microbiome-medication interactions as part of your holistic patient care?
As a healthcare professional, you know that taking a full medical history including medication usage is essential for guiding your patient’s health journey. In terms of microbial health, the usage of antimicrobials such as antibiotics, antifungals and antivirals might alert our attention. However, the relationship between medication usage and microbiome health is far broader, with many medications being known to influence microbiome composition, while in turn, the microbiome influences drug metabolism (see fig 1.)1.
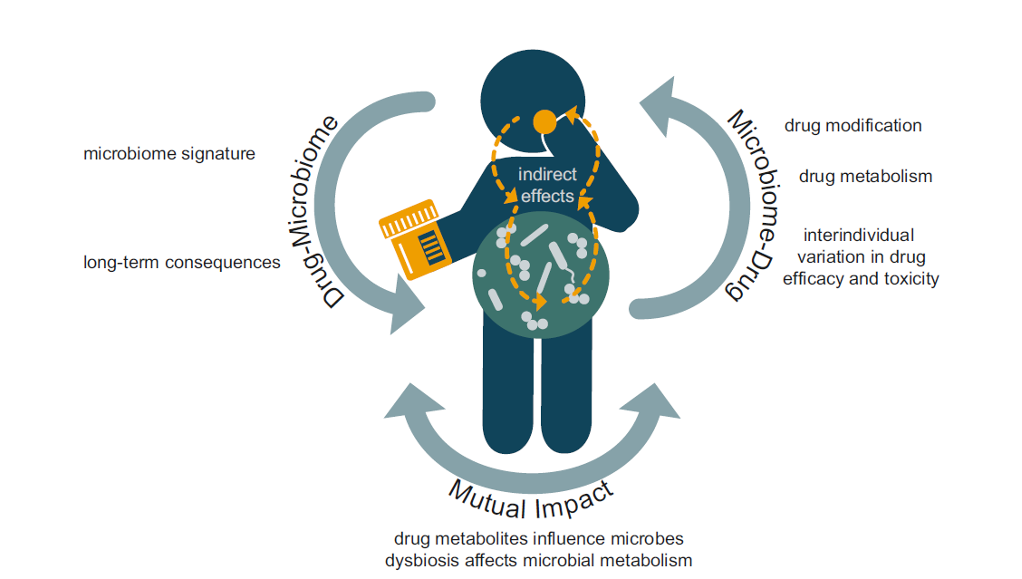
Many medications impact the microbiome (not just antibiotics)2
While the impact of antibiotic use on the gut microbiome has stolen the spotlight, emerging evidence suggests that many commonly prescribed drugs also have unintended consequences for this community of microbes. Known examples include metformin, proton-pump inhibitors (PPIs), nonsteroidal anti-inflammatory drugs (NSAIDs), paracetamol, laxatives, statins, antidepressants and antipsychotics1.
The impact of medications on the microbiome are not necessarily negative and might even be a mechanism of action for therapeutic benefit. For example, studies have suggested that metformin1 and statins3 are associated with beneficial impacts on microbiome composition.
In contrast, it might come as a surprise to learn that protein pump inhibitors (PPIs) have a bigger impact on the gut microbiome than antibiotics4. PPIs, which are widely used to treat conditions such as indigestion, peptic ulcers and acid reflux, are the second most commonly prescribed medication in Australia5. Historically, PPIs have been considered well-tolerated, effective and generally safe, however, concerns have been raised that they are being inappropriately prescribed without due consideration of the potential risks associated with long term usage. PPIs work by reducing stomach acid which can have unintended consequences6. Stomach acid plays numerous roles including aiding the absorption of micronutrients (iron, calcium, vitamin B12) and acting as a defence barrier against invading pathogenic microorganisms. As such, PPI use has been associated with an increased risk of micronutrient deficiencies, gastrointestinal infections and small intestinal bacterial overgrowth (SIBO)2. A recently published systematic review including nearly 900 PPI users has shown that PPI use is associated with distinct alterations in the microbiome, including an over-representation of orally derived species and a decrease in beneficial gut microorganisms.
High beta-glucuronidase potential in the microbiome may increase the impact of certain medications7,8.
Conversely, the microbiome can also influence drug metabolism and help explain the variability in drug responses among individuals. Microbial drug metabolism can lead to drug activation, inactivation or toxicity1.
Beta-glucuronidase is a bacterial enzyme that can limit the excretion of certain medications from the body by reversing a detoxification process known as glucuronidation (see fig. 2).
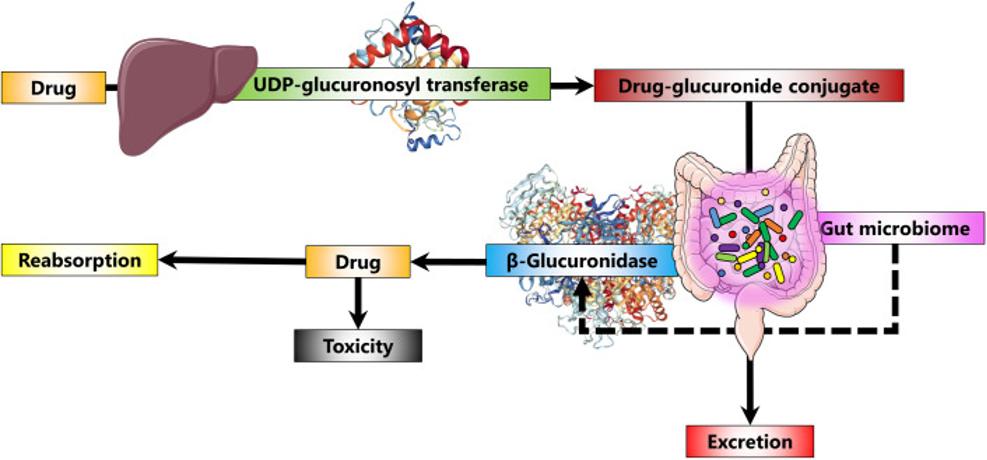
Scientists have recently published a list of 100 medications which are known to be metabolized by glucuronidation and therefore could potentially be reactivated by microbial beta-glucuronidase8. Of these, 22 were for pain management, including commonly used medications such as paracetamol and ibuprofen as well as prescription medications such as morphine. In fact, it is thought that microbial beta-glucuronidase activity is responsible for the intestinal damage that can be associated with nonsteroidal anti-inflammatory drug (NSAID) use9. Other medications identified as being potentially impacted by microbial beta-glucuronidase included those used for diabetes, hormonal therapy, hypercholesterolaemia, hypertension and asthma.
Insight™ gut microbiome analysis reveals the microbiome’s capacity to produce beta-glucuronidase which can be important to consider as it may prevent the excretion of many commonly taken medications. It is important to remember that while metagenomic analysis can provide us with a measure of the whole microbiome’s capacity to produce beta-glucuronidase there are many different types of beta-glucuronidase produced by different bacterial species.
Interested to discuss beta-glucuronidase production potential further? Contact the healthcare team.
To date, 279 different types of microbial beta-glucuronidase enzymes have been identified with each individual microbiome containing from 4 to 38 unique beta glucuronidases9. The difference in structures of microbial beta-glucuronidase influences the predicted location and function of these enzymes within the bacterial species. For example, some enzymes are located within a cell and are able to process smaller compounds that have entered the bacterial cell, while other beta-glucuronidase enzymes are located outside a cell and have the capacity to process larger compounds. There is a common misconception that only “bad” bacteria have the capacity to produce beta-glucuronidase, however, microbial beta-glucuronidases have been identified across a wide variety of species within each phylum in the human gut microbiome9.
Taken together, this highlights the need to consider the microbiome’s beta-glucuronidase potential within the context of your patient’s exposure to certain compounds as well as their presenting symptoms to identify if reducing beta-glucuronidase activity is required. Should this be the case for your patient, the prebiotic fibre, Glucomannan which found in konjac root and konjac-based foods such as low-calorie noodles and pasta, has been shown to reduce faecal beta-glucuronidase activity10.
Summary
The microbiome plays an integral part in human health and must be understood within the context of the individual including their medical history, presenting symptoms, diet, lifestyle and medication usage. Almost all medications have the capacity to alter microbiome composition and function, but these impacts will not always be negative. In turn, the microbiome can impact the effectiveness, tolerance or toxicity of medications. Insight™ gut microbiome analysis utilises world-class metagenomic sequencing to capture a complete picture of the gut microbiome, including your patient’s potential to produce beta-glucuronidase to help you guide their holistic care plan.
As always, if your patient is concerned about how their medications are impacting their microbiome or overall health, it is important that they discuss these concerns with the prescribing practitioner before making any changes to their medication regime.
About the authors

Dr Paula Smith-Brown

Dr Kaylyn Tousignant
References:
- Zimmermann M, Patil KR, Typas A, Maier L. Towards a mechanistic understanding of reciprocal drug-microbiome interactions. Mol Syst Biol, 2021 Mar; 17(3): e10116. https://doi.org/10.15252/msb.202010116.
- Macke L, Schulz C, Koletzko L, Malfertheiner P. Systematic review: the effects of proton pump inhibitors on the microbiome of the digestive tract-evidence from next-generation sequencing studies. Aliment Pharmacol Ther, 2020 Mar; 51(5): 505-526. https://doi.org/10.1111/apt.15604.
- Dias AM, Cordeiro G, Estevinho MM, Veiga R, Figueira L, Reina-Couto M, Magro F; the Clinical Pharmacology Unit, São João Hospital University Centre. Gut bacterial microbiome composition and statin intake-A systematic review. Pharmacol Res Perspect, 2020 Jun; 8(3): e00601. https://doi.org/10.1002/prp2.601.
- Gacesa R, Kurilshikov A, Vich Vila A, et al. The Dutch Microbiome Project defines factors that shape the healthy gut microbiome. bioRxiv, 2020 Nov; https://www.biorxiv.org/content/10.1101/2020.11.27.401125v1
- Australian Government Department of Health. Pharmaceutical Benefits Scheme Expenditure and Prescription Report, 2018 – 2019; www.pbs.gov.au
- Daniels B, Pearson SA, Buckley NA, Bruno C, Zoega H. Long-term use of proton-pump inhibitors: whole-of-population patterns in Australia, Therap Adv Gastroenterol, 2013-2016. 2020; 13:1756284820913743. Published 2020 Mar 19. https://doi.org/10.1177/1756284820913743
- Chackalamannil S, Rotella D, Ward WE. Comprehensive Medicinal Chemistry III. 3rd Amsterdam: Elsevier; 2017. https://www.worldcat.org/title/comprehensive-medicinal-chemistry-iii/oclc/990412964
- Elmassry M, Kim S, Busby B. Predicting drug-metagenome interactions: Variation in the microbial β-glucuronidase level in the human gut metagenomes. Plos One, 2021; https://doi.org/10.1371/journal.pone.0244876
- Pollet RM, D’Agostino EH, Walton WG, Xu Y, Little MS, Biernat KA, Pellock SJ, Patterson LM, Creekmore BC, Isenberg HN, Bahethi RR, Bhatt AP, Liu J, Gharaibeh RZ, Redinbo MR. An Atlas of β-Glucuronidases in the Human Intestinal Microbiome. Structure, 2017 Jul; 25(7): 967-977.e5. https://doi.org/10.1016/j.str.2017.05.003.
- Gao C, Liao MZ, Han LW, Thummel KE, Mao Q. Hepatic Transport of 25-Hydroxyvitamin D3 Conjugates: A Mechanism of 25-Hydroxyvitamin D3 Delivery to the Intestinal Tract. Drug Metab Dispos, 2018; 46(5):581-591. https://doi.org/10.1124/dmd.117.078881