Hydrogen sulphide: why balance is key
Author: Dr Paula Smith Brown
December 2021

Is it time to change your patients’ dietary management of hydrogen sulphide gut levels?
Hydrogen sulphide is a gas that is produced by both gut epithelial cells and the gut microbiome. In turn, gut epithelial cells use hydrogen sulphide as a fuel source, mostly to regulate hydrogen sulphide levels in the colon. Butyrate remains the most important microbially derived energy source for gut cells. Excessive levels of hydrogen sulphide can accumulate in the colon if hydrogen sulphide production exceeds the capacity of gut epithelial cells to utilise it.
At physiological levels, hydrogen sulphide plays an important role in the protection of the gut barrier but at excessive levels, it has been linked to gut barrier dysfunction and inflammation. Advances in the scientific understanding for the interaction between diet and hydrogen sulphide production are leading to an improved understanding to guide the clinical management of patients with a high potential to produce hydrogen sulphide in their gut microbiome.
The effects of high hydrogen sulphide levels
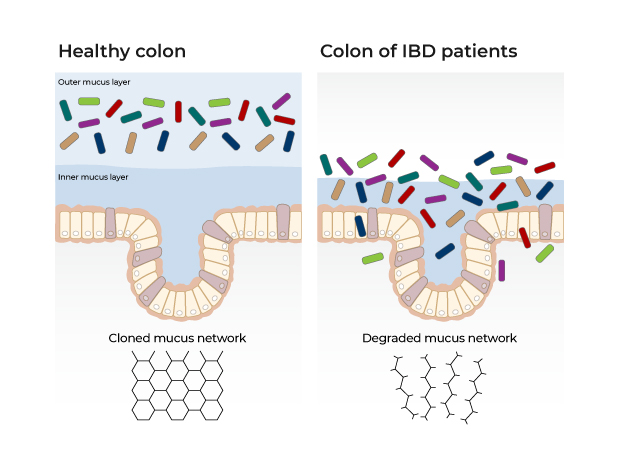
Elevated hydrogen sulphide levels can disrupt gut barrier function and promote inflammation. Raised levels of hydrogen sulphide compromise gut barrier function which can lead to a cycle of inflammation.1-3 Hydrogen sulphide reduces disulphide bonds between mucin molecules which at high levels can compromise the mucus layer lining the gut cells, resulting in reduced protection of gut epithelial cells and increased interaction between the gut microbiome and host immune system.4
In addition, elevated levels of hydrogen sulphide induce oxidative stress and inhibit the utilisation of butyrate by gut cells.1 If prolonged, the resulting starvation of gut cells further reduces their capacity to detoxify hydrogen sulphide and sets up a vicious cycle of escalating hydrogen sulphide levels, gut barrier disruption and inflammation.
Dietary sulphites and hydrogen sulphide
Prior to the widespread utilisation of metagenomic technology, most research focused on the role of sulphate-reducing bacteria in the generation of hydrogen sulphide. Sulphate-reducing bacteria are a small group of bacteria that utilise sulphate obtained from dietary sources or host-derived sulphated mucins. This led to recommendations to reduce dietary sulphates to limit microbial hydrogen sulphide production. Dietary sources of sulphates include sulphur containing metabolites found in the plant genus Allium (e.g. onion, garlic, leek) and cruciferous vegetables (e.g. cabbage, broccoli). In addition, sulphite preservatives are often oxidised to sulphates by the time of consumption. By Australian law, the presence of sulphites must be indicated on the label by code numbers 220 to 228 or the word “sulphite”, and is commonly found in wine, vinegar, cider and dried apricots.
What drives hydrogen sulphide production?
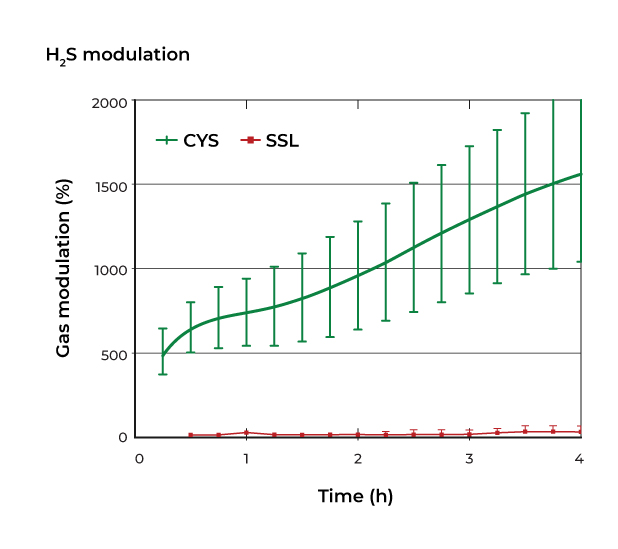
However recent research has revealed that due to their low numbers, sulphate-reducing bacteria make insignificant contributions to overall hydrogen sulphide production by the gut microbiome. In contrast, many bacterial species are able to generate hydrogen sulphide via the fermentation of the sulphur containing amino acids methionine and cysteine. This explains why an in-vitro gas profiling study using human stool showed that sulphates increased hydrogen sulphate production over 4 hours by only 40% while sulphur containing amino acids increased it by 532%.5 We now understand that the sulphur containing amino acids cysteine and methionine are the principal dietary drivers of microbial hydrogen sulphide production.
Utilising metagenomic technology to identify species
The use of metagenomic technology identifies all bacterial species with the ability to produce hydrogen sulphide as well as the overall microbiome’s capacity to produce hydrogen sulphide. If your patient has a high potential to produce hydrogen sulphide in their gut microbiome, we now know that reducing the delivery of sulphur containing amino acids to the microbiome is the most effective way to prevent excessive hydrogen sulphide production. Rich sources of sulphur containing amino acids include fish, eggs and poultry,6 but assessing total protein intake is also of benefit. High intakes of protein saturate the absorptive capacity of the small intestine resulting in increased delivery of protein to the large bowel. 7
What’s best for your patients?
Sulphur containing amino acids are the principal drivers of microbial hydrogen sulphide production, but they are also the principal sources of the essential element sulphur in the diet. Elderly people and those following vegan diets have been shown to be at risk of consuming insufficient quantities of sulphur amino acids. Simultaneously, their dietary requirements for sulphur are increased by the regular use of certain medications, such a paracetamol, which are detoxified in the liver via sulfonation.8 As such it is recommended to balance the need for reducing hydrogen sulphide production with the need for meeting your patients’ sulphur requirements.
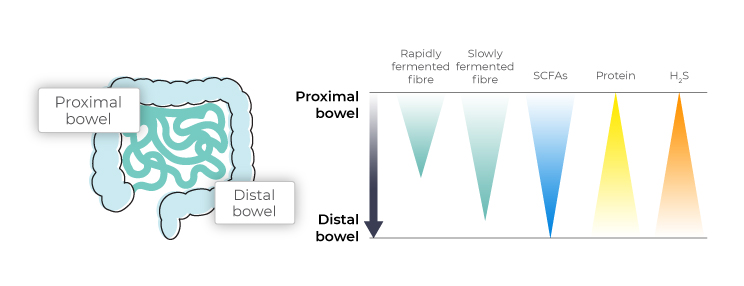
Microbes will only turn to protein fermentation in the absence of sufficient fibre. In the case of a low fibre diet, the sparse existing fibre will be used up in the proximal colon, leaving the microbes in the distal colon to rely on protein and mucin as fuel sources. In particular, the prebiotic fibres resistant starch and fructooligosaccharides (FOS) have been shown to reduce the microbial production of hydrogen sulphide.5
How metagenomic technology can assist your assessments
The use of metagenomic technology allows for the accurate assessment of the relative abundance of hydrogen sulphide producing bacterial species as well as the overall microbiome’s capacity to produce hydrogen sulphide.
Elevated microbial production of hydrogen sulphide can lead to accumulation of toxic levels of hydrogen sulphide in the colon. Limiting the bacterial fermentation of sulphur containing amino acids by increasing fibre intake and preventing excessive protein intake can help prevent excessive hydrogen sulphide production.
To learn more, check out our report tutorial on hydrogen sulphide production.
Find out more about our Food Frequency Questionnaire
About the author

Dr Paula Smith Brown
Paula is a Paediatric Accredited Practising Dietitian with a specialist interest in young child nutrition and growth. She completed a BSC Nutrition and Dietetics (First Class Honours) at Kings College London, in 2002, and was awarded the Maud Taylor Prize for best overall student. She has had a diverse career including clinical, community, industry and NGO roles internationally.
References:
- Teigen LM, Geng Z, Sadowsky MJ, Vaughn BP, Hamilton MJ, Khoruts A. Dietary Factors in Sulfur Metabolism and Pathogenesis of Ulcerative Colitis. Nutrients. 2019 Apr 25;11(4):931. doi: 10.3390/nu11040931. PMID: 31027194; PMCID: PMC6521024.
- Nguyen LH, Ma W, Wang DD, Cao Y, Mallick H, Gerbaba TK, Lloyd-Price J, Abu-Ali G, Hall AB, Sikavi D, Drew DA, Mehta RS, Arze C, Joshi AD, Yan Y, Branck T, DuLong C, Ivey KL, Ogino S, Rimm EB, Song M, Garrett WS, Izard J, Huttenhower C, Chan AT. Association Between Sulfur-Metabolizing Bacterial Communities in Stool and Risk of Distal Colorectal Cancer in Men. Gastroenterology. 2020 Apr;158(5):1313-1325. doi: 10.1053/j.gastro.2019.12.029. Epub 2020 Jan 20. PMID: 31972239; PMCID: PMC7384232.
- Baldini F, Hertel J, Sandt E, Thinnes CC, Neuberger-Castillo L, Pavelka L, Betsou F, Krüger R, Thiele I; NCER-PD Consortium. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020 Jun 9;18(1):62. doi: 10.1186/s12915-020-00775-7. PMID: 32517799; PMCID: PMC7285525.
- Ljssennagger N, Belzer C, Hooiveld GJ, Dekker J, van Mil SW, Müller M, Kleerebezem M, van der Meer R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci U S A. 2015 Aug 11;112(32):10038-43. doi: 10.1073/pnas.1507645112. Epub 2015 Jul 27. PMID: 26216954; PMCID: PMC4538683.
- Yao CK, Rotbart A, Ou JZ, Kalantar-Zadeh K, Muir JG, Gibson PR. Modulation of colonic hydrogen sulfide production by diet and mesalazine utilizing a novel gas-profiling technology. Gut Microbes. 2018 Nov 2;9(6):510-522. doi: 10.1080/19490976.2018.1451280. Epub 2018 May 9. PMID: 29561196; PMCID: PMC6287689.
- Doleman JF, Grisar K, Van Liedekerke L, Saha S, Roe M, Tapp HS, Mithen RF. The contribution of alliaceous and cruciferous vegetables to dietary sulphur intake. Food Chem. 2017 Nov 1;234:38-45. doi: 10.1016/j.foodchem.2017.04.098. Epub 2017 Apr 18. PMID: 28551250; PMCID: PMC5460521.
- Blachier F, Andriamihaja M, Larraufie P, Ahn E, Lan A, Kim E. Production of hydrogen sulfide by the intestinal microbiota and epithelial cells and consequences for the colonic and rectal mucosa. Am J Physiol Gastrointest Liver Physiol. 2021 Jan 1;320(2):G125-G135. doi: 10.1152/ajpgi.00261.2020. Epub 2020 Oct 21. PMID: 33084401.
- Nimni ME, Han B, Cordoba F. Are we getting enough sulfur in our diet? Nutr Metab (Lond). 2007 Nov 6;4:24. doi: 10.1186/1743-7075-4-24. PMID: 17986345; PMCID: PMC2198910.